Introduction: Infections continue to be one of the main causes of morbidity and mortality in multiple myeloma (MM) patients. Compared to the general population, MM patients have a 7-fold increased risk of bacterial infections and 10-fold risk of viral infections. The recent National Comprehensive Cancer Network (NCCN) guideline has provided guidance for prevention of cancer-related infections; however, utilization of anti-infective prophylaxis among MM patients in real-world practice has not been fully established. Daratumumab, a human IgGκ monoclonal antibody targeting CD38, is approved as monotherapy and in combination with standard-of-care regimens for newly diagnosed MM (NDMM) and relapsed/refractory MM (RRMM). The primary objective of the study was to describe the proportion of MM patients who received anti-infective prophylaxis when initiating a daratumumab-based treatment regimen.
Methods: Medical and pharmacy claims from the Optum's de-identified Clinformatics® Data Mart Database were obtained for a cohort of US patients with NDMM or RRMM who initiated treatment with daratumumab (in combination with other antimyeloma agents or monotherapy) between 1/1/2017 and 12/24/2019. The index date was defined as the first daratumumab treatment date in the study period. Two steps were applied to identify anti-infective prophylaxis. First, use of anti-infective prophylaxis was assessed during the period between the week prior to and the week after the date of daratumumab initiation, including anti-virals for preventing herpesviruses (i.e., herpes zoster, herpes simplex, and cytomegalovirus), systemic anti-bacterials, systemic anti-fungals, Pneumocystis jiroveci pneumonia (PJP) prophylaxis, and intravenous/subcutaneous immune globulin (IVIG/SCIG). Second, use of an anti-infective drug was defined as prophylactic if there was no diagnosis for an infection treated by the anti-infective drug (i.e., patients with infection events one week prior to and one day after the start of drug prescription were excluded). Receipt of vaccines for seasonal influenza (in the year prior to daratumumab initiation) and herpes zoster and pneumococcal infections (at any time prior in the patient's claims history) were also assessed.
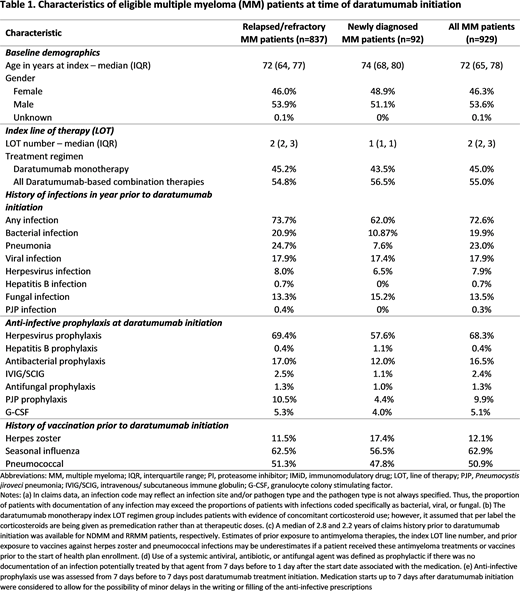
Results: 929 newly daratumumab-treated patients were eligible for study inclusion; 92 had NDMM (median age 74 years; 48.9% female) and 837 had RRMM (median age 72 years; 46.0% female). At the time of daratumumab initiation, antibacterial prophylaxis was administered to 12.0% of NDMM and 17.0% of RRMM patients; antifungal prophylaxis to 1.1% of NDMM and 1.3% of RRMM patients; and PJP prophylaxis to 4.4% of NDMM and 10.5% of RRMM patients (Table 1). IVIG/SCIG prophylaxis was given to 1.1% of NDMM patients and 2.5% of RRMM patients. Herpesvirus prophylaxis was administered to 57.6% of NDMM and 69.4% of RRMM patients, and 17.4% of NDMM and 11.5% of RRMM patients received vaccination for herpes zoster. Seasonal influenza and pneumococcal vaccinations were given to 56.5% and 47.8% of NDMM patients and 62.5% and 51.3% of RRMM patients, respectively.
Conclusion: Use of herpesvirus prophylaxis was approximately 70% in RRMM and 60% in NDMM patients at time of daratumumab initiation, with limited use of herpes zoster vaccination, suggesting that a meaningful minority of patients may not be protected against herpesvirus as recommended by NCCN guidelines for patients treated for MM. While rates of influenza and pneumococcal vaccination were higher than for herpes zoster, nearly half of patients did not have evidence of vaccination for these two pathogens in their claims data. These results indicate that a significant percentage of MM patients are not receiving care to optimally prevent potential viral infections. Further studies are needed to understand the potential benefit of infectious prophylaxis in the clinical management of MM patients.
Tai:Janssen Scientific Affairs: Current Employment. Ammann:Janssen Scientific Affairs: Current Employment, Current equity holder in publicly-traded company. Kaila:Janssen Scientific Affairs: Current Employment. Pericone:Janssen Scientific Affairs: Current Employment, Current equity holder in publicly-traded company. Singh:Mu Sigma: Current Employment, Other: Mu Sigma was contracted by Janssen Scientific Affairs to conduct the analyses for the present study. Lin:Janssen Scientific Affairs: Current Employment, Current equity holder in publicly-traded company. Davies:Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotech: Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal